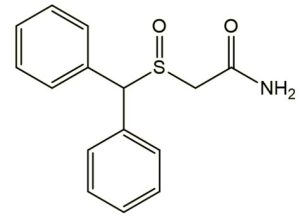
Modafinil, sold under the brand name Provigil among others, is a central nervous system (CNS) stimulant medication used to treat sleepiness due to narcolepsy, shift work sleep disorder, and obstructive sleep apnea.It is sometimes prescribed off-label for treating Attention deficit hyperactivity disorder (ADHD) in children, having no benefit in ADHD for adults. While it has seen off-label use as a purported cognitive enhancer to improve wakefulness, in animal and human studies the research on its effectiveness for this use is inconclusive. Modafinil is taken by mouth. It comes in oral tablet form of 100mg or 200mg. Clinical studies have not found evidence of diminished response or tolerance to modafinil’s wakefulness-promoting properties at therapeutic doses over prolonged periods. In addition to its medical uses, modafinil has gained popularity among students, office workers, professionals, and others who seek enhanced wakefulness and cognitive function. However, studies have shown mixed results regarding its cognitive enhancement abilities.
While modafinil is generally well-tolerated when used according to proper dosing guidelines, it does carry potential risks and side effects that should be considered before use. Additionally, there are concerns about the potential abuse of modafinil due to its stimulant-like properties. Modafinil’s side effects include headaches, anxiety, and nausea. Serious side effects in high doses include delusions, unfounded beliefs, paranoia, irrational thought, and transient depression, possibly due to its effects on dopamine receptors in the brain, as well as allergic reactions. The amount of medication used should be adjusted in those with kidney problems, as this medication has markedly increased side effects during renal insufficiency. It is not recommended in those with an arrhythmia, significant hypertension, or left ventricular hypertrophy.Modafinil appears to work by acting on dopamine and modulating the areas of the brain involved with the sleep cycle.
Originally developed in the 1970s by French neuroscientist Michel Jouvet and Lafon Laboratories, Modafinil has been prescribed in France since 1994, and was approved for medical use in the United States in 1998.Its legal status varies by jurisdiction; in the United States it is classified as a schedule IV controlled substance, whereas in the United Kingdom it is a prescription only medication. In some countries no controls apply. It is available as a generic medication. In 2020, modafinil was the 302nd most commonly prescribed medication in the United States, with just over 1000000 prescriptions.There are ongoing debates around using modafinil as a performance-enhancing drug or “smart drug”, especially outside of medically approved uses like treating sleep disorders or ADHD. Some view its usage negatively due to ethical concerns about unfair advantages or reliance on drugs for improved productivity

Reviews
There are no reviews yet.