Vitamin D is a group of fat-soluble secosteroids responsible for increasing intestinal absorption of calcium, magnesium, and phosphate, and for many other biological effects.In humans, the most important compounds in this group are vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol).
The major natural source of vitamin D is synthesis of cholecalciferol in the lower layers of the epidermis of the skin, through a photochemical reaction with Ultraviolet B (UV-B) radiation from sun exposure or UV-B lamps. Cholecalciferol and ergocalciferol can be ingested from the diet and supplements. Only a few foods, such as the flesh of fatty fish, naturally contain significant amounts of vitamin D.In the U.S. and other countries, cow’s milk and plant-derived milk substitutes are fortified with vitamin D, as are many breakfast cerealsMushrooms exposed to ultraviolet light contribute useful amounts of vitamin D2.Dietary recommendations typically assume that all of a person’s vitamin D is taken by mouth, because sun exposure in the population is variable and recommendations about the amount of sun exposure that is safe are uncertain in view of the skin cancer risk
Vitamin D from the diet, or from skin synthesis, is biologically inactive. It is activated by two protein enzyme hydroxylation steps, the first in the liver and the second in the kidneys.Because vitamin D can be synthesized in adequate amounts by most mammals if they get enough sunlight, it is not essential and therefore is technically not a vitamin.[3] Instead it can be considered a hormone, with activation of the vitamin D pro-hormone resulting in the active form, calcitriol, which then produces effects via a nuclear receptor in multiple locations.
Cholecalciferol is converted in the liver to calcifediol (25-hydroxycholecalciferol); ergocalciferol is converted to 25-hydroxyergocalciferol.These two vitamin D metabolites (called 25-hydroxyvitamin D or 25(OH)D) are measured in serum to determine a person’s vitamin D status. Calcifediol is further hydroxylated by the kidneys and some of the immune system cells to form calcitriol (1,25-dihydroxycholecalciferol), the biologically active form of vitamin D Calcitriol circulates as a hormone in the blood, having a major role regulating the concentration of calcium and phosphate, and promoting the healthy growth and remodeling of bone. Calcitriol also has other effects, including some on cell growth, neuromuscular and immune functions, and reduction of inflammation.
Vitamin D has a significant role in calcium homeostasis and metabolism.Its discovery was due to effort to find the dietary substance lacking in children with rickets (the childhood form of osteomalacia). Vitamin D supplements are given to treat or to prevent osteomalacia and rickets.The evidence for other health effects of vitamin D supplementation in vitamin D–replete individuals is inconsistent. The effect of vitamin D supplementation on mortality is not clear, with one meta-analysis finding a small decrease in mortality in elderly people. Except for the prevention of rickets and osteomalacia in high-risk groups, any benefit of vitamin D supplements to musculoskeletal or general health may be small.
Types
| Name | Chemical composition | Structure |
|---|---|---|
| Vitamin D1 | Mixture of molecular compounds of ergocalciferol with lumisterol, 1:1 | |
| Vitamin D2 | ergocalciferol (made from ergosterol) | 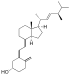 |
| Vitamin D3 | cholecalciferol(made from 7-dehydrocholesterol in the skin). |  |
| Vitamin D4 | 22-dihydroergocalciferol |  |
| Vitamin D5 | sitocalciferol(made from 7-dehydrositosterol) |  |
Several forms (vitamers) of vitamin D exist. The two major forms are vitamin D2 or ergocalciferol, and vitamin D3 or cholecalciferol. Vitamin D without a subscript refers to either D2 or D3, or both, and is known collectively as calciferol.
Vitamin D2 was chemically characterized in 1931. In 1935, the chemical structure of vitamin D3 was defined and shown to result from the ultraviolet irradiation of 7-dehydrocholesterol. A chemical nomenclature for vitamin D forms was recommended in 1981, but alternative names remain in common use.
Chemically, the various forms of vitamin D are secosteroids, that is, steroids in which one of the bonds in the steroid rings is broken. The structural difference between vitamin D2 and vitamin D3 is in the side chain, which contains a double bond, between carbons 22 and 23, and a methyl group on carbon 24 in vitamin D2. Many vitamin D analogues have been synthesized.